Author Guideline
General Terms of Publication
A. YOUR ARTICLE
1. Preparing Your Manuscript
All authors submitting to Laboratory Journal of Infectious Diseases (LJID) should confirm to the Uniform Requirements for Manuscripts Submitted to LJID Journals. The manuscripts submitted to LJID should be written in English.
Articles that have been published previously in another language, or in a journal that is not widely available, may be submitted for consideration provided the editor of the source journal and the copyright older give written approval and the origin of the article is declared in the manuscript. Originality Statement Form approvals must be included with the submitted manuscript
2. Word Limits
Please include a word count for your paper. Although there is no page limit for a Regular Paper, it is strongly suggested that a complete manuscript should normally be between 4,000-8,000 words.
3. Manuscript Format
All manuscript format is MS word. Please use academic English style consistently throughout your manuscript. Figures (illustrations) and tables should be put separately from the text. Each manuscript should be typed single-spaced on A4 (8.5” x 11”) paper size. Articles should be written using our template.
4. Subscription details
Frequency: 2 issues per year
Subscription period: one year
B. BEFORE SUBMITTING
1. Approval
Ensure all authors have seen and approved the final version of the article prior to submission. All authors must also approve the journal you are submitting to.
2. Publication Charges
Since Juni 1, 2025, Laboratory Journal Infectious Diseases (LJID) currently has no Article Publication Charges (APC). Submitting, processing, and publishing articles in our journal is completely free of charge.
C. UPLOADING YOUR SUBMISSION
1. Cover Letter
Upload a cover letter as a separate file in the online system. The length limit is 1 page. The cover letter template file could be downloaded at This Link
2. Originality Statement Form
Fill out this form to confirm that neither the manuscript nor any parts of its content are currently under consideration or published in another journal. All author signs the form and send it together with cover letter and manuscript. Make sure you have followed our template.
3. Title Page
a. Title
Title page should contain the title of the paper in bold face, title case (font size 16), names of the authors in normal face, upper case (font size 12) followed by address in normal face lower case. The author to whom all correspondence addressed should be denoted by an asterisk mark. The title should be as short as possible and precisely indicate the nature of the work in the communication. Names of the authors should appear as initials followed by surnames. At the bottom of the left corner of the title page, please mention “*Address For correspondence” and provide a functional e-mail address. The address of the corresponding author to whom all correspondence may be sent should be given only if it is different from the address already given under authors’ names.
The title should accurately, clearly, and concisely reflect the emphasis and content of the paper. The title must be brief and grammatically correct. The titles should not normally include numbers, acronyms, abbreviations or punctuation. They should include sufficient detail for indexing purposes but please be general enough for readers outside the field to appreciate what the paper is about. Avoid specialist abbreviations if possible. Titles are no more than 10–12 words. Make sure you have followed our template
b. Author list
All authors must be listed on the title page and entered on the Manuscripts submission in the correct order. Ensure all author email addresses provided are valid.
4. Manuscript with Following Guidelines
a. Title
Concise and informative. Titles are often used in information-retrieval systems. Avoid abbreviations and formulae where possible.
b. Abstract
We strongly encourage authors to use the following style of structured abstracts, but without headings:
- Background: Place the question addressed in a broad context and highlight the purpose of the study;
- Methods: briefly describe the main methods or treatments applied;
- Results: summarize the article’s main findings;
- Conclusions: indicate the main conclusions or interpretations
The abstract should be an objective representation of the article and it must not contain results that are not presented and substantiated in the main text and should not exagger-ate the main conclusions. Do not include abbreviations and citations. The abstract contains of 200-250 words. Avoid specialist abbreviations.
- Abbreviations should be defined in parentheses the first time they appear in the abstract, main text, and in figure or table captions and used consistently thereafter.
- SI Units (International System of Units) should be used. Imperial, US customary and other units should be converted to SI units whenever possible.
- Accession numbers of RNA, DNA and protein sequences used in the manuscript should be provided in the Materials and Methods section. Also see the section on Deposition of Sequences and of Expression Data.
c. Keywords
Three to five keywords after abstract. Read making your article more discoverable, including information on choosing a title and search engine optimization.
d. Introduction
The introduction should briefly place the study in a broad context and highlight why it is important. It should define the purpose of the work and its significance. The current state of the research field should be carefully reviewed and key publications cited. Please highlight controversial and diverging hypotheses when necessary. Finally, briefly mention the main aim of the work and highlight the principal conclusions. As far as possible, please keep the introduction comprehensible to scientists outside your particular field of research. References should be numbered in order of appearance and indicated by a numeral or numerals in square brackets—e.g., (1) or (2,3), or (4–6). See the end of the document for further details on references.
e. Materials and methods
This section should describe the equipment and materials utilized and the manners in which the work was conducted. Sufficient information should be provided to enable peers to accurately replicate the work. Detailed experimental methods must be included in this section, and not just as a figure legend. Standard laboratory procedures need not be described in detail, but reference citation is necessary. Ethical considerations should be described in this section when necessary.
Describe the materials used in the experiment, year of the experimentation, enough details that a competent researcher could repeat your experiment. The materials and method should not be listed separately. For commercial sources of used materials, the name of the company, and the town and country in which they are located should be indicated.
If you have more than one method, use subsections with relevant headings, e.g. different models, in vitro and in vivo studies, statistics, material and reagents, etc. Methods already published should be indicated by a reference, with only the relevant modifications described here. e.g., “… The method was referring to centrifugation at room temperature; we modified it (9) for the protection of fragile DNA pellet during further extraction steps….”. Submission of sequence data to databases: Novel nucleotide or protein sequence data must be deposited in the GeneBank, EMBL or DDBJ databases and an accession number obtained before the paper could be accepted for publication.
Methods sections describing research using human or animal subjects and/or tissue or field sampling must include required ethics statements. Methods sections describing research using cell lines must state the origin of the cell lines used. Specify the computer software used.
In general, LJID adheres to the International System of Units (SI) for how units of measurements are written. Use SI symbols, give concentrations in mol/L and define the term % as w/v or v/v for all solutions. For international units use IU (U should be used for enzyme activity). Describe statistical methods with enough detail to enable a knowledgeable reader with access to the original data to verify the reported results. Specify the type of equipment (microscopes/objective lenses, cameras, detectors) used to obtain images. When possible, quantify findings and present them with appropriate indicators of measurement error or uncertainty (such as confidence intervals).
Abbreviations should be defined fully only at first mention and used consistently thereafter. Species name is writing in italics (e.g., Helicobacter phylori). Scientific name with the authority should be given in the title and the first time the species is mention in the text. Thereafter, either the vernacular of common name of the species or the shortened scientific name (e.g., M. tuberculosis, M. leprae) can be used, but not a mixture of both).
2.1. Sub Section
Xxxx
2.2. Sub Section
Xxxx
Ethics of Human and Animal Experimentation
- Human Subject Research. The Author should ensure that studies involving experiments on humans must have Institutional Review Board (IRB) and/or national research ethics committee approval. Manuscripts should include a statement that the informed consent was obtained for experimentation with human subjects and that it conforms to standards currently applied in the country of origin. The certificate of ethical research should be attached. The name of the authorizing body should be stated in the paper. The privacy rights of human subjects must always be observed.
- Animal Research. The Author should ensure that studies involving experiments on animals must include a statement in the manuscript indicating that the international, national, and/or institutional guide lines for the care and use of animals have been followed, and that the study has been approved by a research ethics committee. Procedures should be such that experimental animals do not have to suffer unnecessarily. Papers should include details of the procedures and of anaesthetics used.
- A fully informed written consent which should be documented in the paper
All individuals have individual rights that are not to be infringed. Individual participants in studies have the right to decide what happens to the identifiable data gathered from them during a study. Hence, it is important that all participants gave their informed consent in writing prior to inclusion in the study. The manuscripts that include information about participants, written informed consent for the publication of these must be obtained from all the participants.
f. Results
This part focus on the fulfilment of stated objectives as given in the introduction. Results and discussion are separate sections. It should contain the findings presented in the form of figures and figures. Provide a concise and precise description of the experimental results, their interpretation as well as the experimental conclusions that can be drawn.
This section may be divided by subheadings. It should provide a concise and precise description of the experimental results, their interpretation, as well as the experimental conclusions that can be drawn.
3.1. Sub Section
Xxxx
3.2. Sub Section
Xxxx
3.3. Formatting of Mathematical Components
This is example 1 of an equation:
a = 1,
The text following an equation need not be a new paragraph. Please punctuate equations as regular text.
This is example 2 of an equation:
a = b + c + d + e + f + g + h + i + j + k + l + m + n + o + p + q + r + s + t + u + v + w + x + y + z
The text following an equation need not be a new paragraph. Please punctuate equations as regular text.
Figures
- Please submit graph as editable text and not as images.
- Figures should be high quality (12000 dpi for line art, 600 dpi for grayscale and 300 dpi for color, at the correct size).
- Figures should be supplied in one of our preferred file formats: JPEG, JPG or PNG. The aim of the figure legend should be to describe the key messages of the figure, but the figure should also be discussed in the text.
- Number figures in the order they are first mentioned in text. Do not write “the figure above” or “the figure below.”
- Each legend should have a concise title of no more than 15 words. The legend itself should be succinct, while still explaining all symbols and abbreviations. Avoid lengthy descriptions of methods. Put the legend inside the figure box, preferably above or to the right of the figure.
- The style of the graphs and charts and the size and appearance of letters and numbers should be consistent within a paper.
- Do not draw a box around line-art figures. Multipaneled figures should be labelled (lowercase a, b, c, etc.) and combined into one file.
- The graphic elements: Do not crowd the interval marks on axis scales. Legend includes identify symbols, lines, and patterns. Put the legend inside the figure box, preferably above or to the right of the figure.
Figure 1. This is a figure. Schemes follow another format. If there are multiple panels, they should be listed as: (a) Description of what is contained in the first panel; (b) Description of what is contained in the second panel. Figures should be placed in the main text near to the first time they are cited.
Tables
- Tables should present new information rather than duplicating what is in the text. Regarded should be able to interpret table without reference to the text. Print screen is not allowed.
- Use Table’s title with sentence-style capitalization (only the first word has an initial capital). Use only lowercase for legends and for units of measure. Define all abbreviations in the caption, even if they appear in the overall abbreviations list
- Number tables in the order they are first mentioned in text. Do not write “the table above” or “the table below.”
- Always use Microsoft Word's table feature. DO NOT create tables by using the space bar and/or tab keys. Do not submit tables in Microsoft Excel.
- Do not use the enter key within the body of the table. Instead, separate data horizontally with a new row.
- Do not insert blank columns or rows.
- Asterisks or letters next to values indicating statistical significance should appear in the same cell as the value, not an adjacent cell (i.e., they should not have their own column).
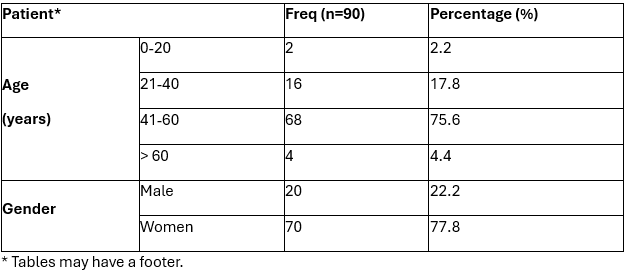
Table 1. Data on age range and gender post-craniotomy patients at the Jemursari Islamic Hospital, Surabaya, 2018.
Ensure that each FIGURE OR TABLE has a caption. Supply captions separately, not attached to the figure. A caption should comprise a brief title (not on the FIGURE OR TABLE itself) and a description of the illustration. Keep text in the FIGURE OR TABLE themselves to a minimum but explain all symbols and abbreviations used.
g.Discussion
This section should not repeat the description from the Introduction and the Results sections, but rather provide an interpretation of the results. Authors should address controversial or unresolved points, results obtained by other researchers, and the evidence available for drawing conclusions.
Discussion section should present comprehensive analysis of the results in the light of any previous research. Discussion may also be combined with results. The Discussion should spell out the major conclusions and interpretations of the work including some explanation on the significance of these conclusions. How do the conclusions affect the existing assumptions and models in the field? How can future research build on these observations? What are the key experiments that must be done? The Discussion should be concise and tightly argued.
4.1 Interpretation of Key Findings
In this study, our findings revealed [summarize main results]. The observed [increases/decreases] in [specific outcomes] within the experimental group suggest [potential explanations] (Hafid et al., 2022; Nurfatimah et al., 2021). These results are consistent with previous research by [cite relevant studies] and support the notion that [provide an overarching interpretation]. Notably, our study contributes to the existing evidence base by [highlighting any unique aspects or novel findings] (Amsal et al., 2021; Firaningsih, Sitorus, Nurfatimah, Longgupa, & Ramadhan, 2021; Ramadhan, Maradindo, Nurfatimah, & Hafid, 2021).
4.2 Comparison with Previous Studies
Comparing our results with those of previous studies is essential for contextualization. The findings of [cite relevant studies] are in line with our results, confirming the robustness of the observed [patterns/trends]. However, disparities with [other studies] warrant consideration. Possible explanations for these discrepancies include [discuss potential reasons], emphasizing the need for further research to reconcile conflicting findings in the literature (Mulia Sakti, Hi. Amir Sene, & Ramadhan, 2024; Taqwin et al., 2023).
4.3 Implications for Public Health
The implications of our study extend to the realm of public health by [discuss how the findings may impact public health practices or policies] (Maryani et al., 2024; Staryo et al., 2024). For instance, the observed [improvements/worsening] in [specific outcomes] suggest potential interventions to enhance [healthcare practices/public health strategies]. Our results align with the broader goals outlined in [relevant global health initiatives], emphasizing the international relevance of our findings.
4.4 Limitations and Cautions
Despite the meaningful contributions, our study has several limitations that warrant acknowledgment. [Discuss study limitations, such as sample size constraints, potential biases, or methodological challenges]. These limitations may have influenced the generalizability of our findings and should be considered in the interpretation of results. Future research should address these limitations to further refine our understanding of [health issue].
4.5 Recommendations for Future Research
Building on the insights gained from this study, future research should focus on [identify specific areas for further investigation]. Addressing the limitations identified in this study, such as [mention limitations], will contribute to a more comprehensive understanding of [health issue]. Additionally, exploring [related aspects] may unveil new dimensions and nuances that were beyond the scope of our study.
h. Conclosion
Conclusion section should bring out the significance of your research paper, show how you’ve brought closure to the research problem, and point out remaining gaps in knowledge by suggesting issues for further research
i. Author contributions
For research articles with several authors, a short paragraph specifying their individual contributions must be provided. The following statements should be used “Con-ceptualization, X.X. and Y.Y.; methodology, X.X.; software, X.X.; validation, X.X., Y.Y. and Z.Z.; formal analysis, X.X.; investigation, X.X.; resources, X.X.; data curation, X.X.; writing—original draft preparation, X.X.; writing—review and editing, X.X.; visualization, X.X.; supervision, X.X.; project administration, X.X.; funding acquisition, Y.Y. All authors have read and agreed to the published version of the manuscript.” Please turn to the CRediT taxonomy for the term explana-tion. Authorship must be limited to those who have contributed substantially to the work report-ed.
j.Funding
Please add: “This research received no external funding” or “This research was funded by NAME OF FUNDER, grant number XXX” and “The APC was funded by XXX”. Check careful-ly that the details given are accurate and use the standard spelling of funding agency names at https://search.crossref.org/funding. Any errors may affect your future funding.
k. Acknowledgments
List here those individuals who provided help during the research (e.g., providing language help, writing assistance or proofreading the article, etc.). Financial support, including grants, fellowships, and scholarships should appear in this section.
l. Ethics statement
None. Studies involving humans and animals must have been performed with the approval of an appropriate ethics committee and provide the reference number.
m. Conflict of interest
All authors must disclose any commercial or other association (e.g., pharmaceutical stock ownership, consultancy, advisory board membership, travel grants, relevant patents, or research funding) that might present a conflict of interest regarding the manuscript submitted. The corresponding author is responsible for obtaining the relevant information from all co-authors. Appropriate declarations or “None to declare” should appear at the end of the text under the subheading “Conflict of interest.”
n. Funding
List funding sources in this standard way to facilitate compliance to funder's requirements. It is not necessary to include detailed descriptions on the program or type of grants and awards. When funding is from a block grant or other resources available to a university, college, or other research institution, submit the name of the institute or organization that provided the funding. If no funding has been provided for the research, please include the following sentence: “This study did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.”
n. References
References should include only articles that are published in indexed international journals. LJID uses “APA” style, as outlined in the Mendeley sample references and include a DOI number. Place the author's last name and the year of publication in parentheses, like (Smith, 2020). A complete list of all sources referenced in the text, placed at the end of the paper. Alphabetical Order: Entries are listed alphabetically by the author's last name. Verify all references prior to submission. Identify references in text, tables and legends by Arabic numerals in superscript. References cited only in tables or figure legends should be numbered in accordance with the sequence established by the first identification in the next of the particular table or figure. The total number of references in the research article is recommended to be equal or more than 20 and they must be less than 5 years old (80% of total reference). Do not cite unavailable and unpublished work.
|
Journal Articles
|
Kambuno, N. T., Putra, A. G. A., Louisa, M., Wuyung, P. E., Timan, I. S., Silaen, O. S. M., ... & Supali, T. (2025). Moringa oleifera Leaf Extract Improves Cognitive Function in Rat Offspring Born to Protein-Deficient Mothers. Biomedicines, 13(2), 346. |
|
A Book
|
Strogatz SH. Nonlinear dynamics and chaos. Reading (MA): Perseus Books Publishing; 1994. https://doi.org/10.1201/9780429492563 |
|
A Chapter in Authored Book
|
Riffenburgh RH. Statistics in medicine. 2nd ed. Amsterdam (Netherlands): Elsevier Academic Press; 2006. Chapter 24, Regression and correlation methods; p. 447–486. https://doi.org/10.1016/B978-0-12-088770-5.X5036-9 |
|
A Chapter in Edited Book
|
Sumner P, Mollon JD. Did primate trichromacy evolve for frugivory or folivory? In: Mollon JD, Pokorny J, Knoblauch K, editors. Normal and defective colour vision. New York (NY): Oxford University Press; 2003. p. 21–30. https://doi.org/10.1093/acprof:oso/9780198525301.003.0003 |
|
Conference |
Diaz J, Gonzalez C, Escalona O. Nonlinear analysis of the ECG during atrial fibrillation in patients for low energy internal cardioversion. Proceedings of the 30th Annual International Conf Proc IEEE Eng Med Biol Soc. 2008; 2008:1619–1622. https://doi.org/ 10.1109/IEMBS.2008.4649483 |
Privacy Statement
The names and email addresses entered in this journal site will be used exclusively for the stated purposes of this journal and will not be made available for any other purpose or to any other party.
Suggesting reviewers
Please submit the names and institutional e-mail addresses of several potential reviewers.
You should not suggest reviewers who are colleagues, or who have co-authored or collaborated with you during the last three years. Editors do not invite reviewers who have potential competing interests with the authors. Further, in order to provide a broad and balanced assessment of the work, and ensure scientific rigor, please suggest diverse candidate reviewers who are located in different countries/regions from the author group. Also consider other diversity attributes e.g. gender, race and ethnicity, career stage, etc. Finally, you should not include existing members of the journal's editorial team, of whom the journal are already aware.
Note: the editor decides whether or not to invite your suggested reviewers.
D. AFTER ACCEPTANCE
1. Proofs
To ensure a fast publication process of the article, we kindly ask authors to provide us with their proof corrections within two days. Corresponding authors will receive an e-mail with a link to our online proofing system, allowing annotation and correction of proofs online. The environment is similar to MS Word: in addition to editing text, you can also comment on figures/tables and answer questions from the Copy Editor. Web-based proofing provides a faster and less error-prone process by allowing you to directly type your corrections, eliminating the potential introduction of errors.
If preferred, you can still choose to annotate and upload your edits on the PDF version. All instructions for proofing will be given in the e-mail we send to authors, including alternative methods to the online version and PDF.
We will do everything possible to get your article published quickly and accurately. Please use this proof only for checking the typesetting, editing, completeness and correctness of the text, tables and figures. Significant changes to the article as accepted for publication will only be considered at this stage with permission from the Editor. It is important to ensure that all corrections are sent back to us in one communication. Please check carefully before replying, as inclusion of any subsequent corrections cannot be guaranteed. Proofreading is solely your responsibility.
2. Proofs
The corresponding author will be provided with a PDF file of the article via e-mail. For an extra charge, paper offprints can be ordered.
Juni 01, 2025
Editor-in-Chief
Laboratory Journal Infectious Diseases
E-mail: normatiku@poltekeskupang.ac.id




